Table of Contents
Vaccines under development
- There are already at least 254 therapiesand 95 vaccines related to Covid-19 being explored.
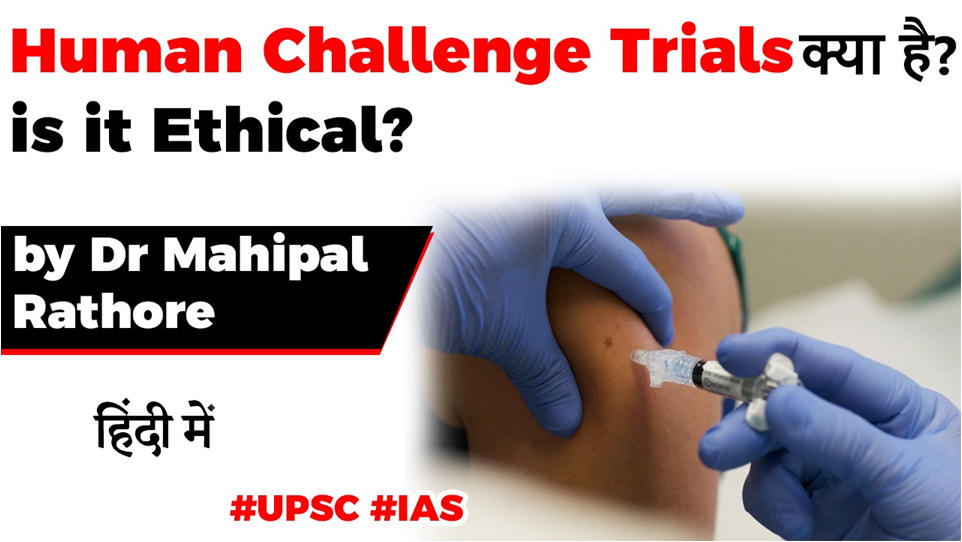
- The Human challenge method, which involves intentionally infecting volunteers with the novel coronavirus, is being promoted in order to speed up the process of preparing a vaccine.


What are the steps in the development of a new Vaccine?
- The general stages of the development cycle of a vaccine are
- Exploratory stage
- Pre-clinical stage
- Clinical development
- Regulatory review and approval
- Manufacturing
- Quality control
Clinical stages
- Phase 1 – small groups of people receive the trial vaccine.
- Phase 2 – the clinical study is expanded and the vaccine is given to people who have characteristics such as age and physical health, similar to those for whom the new vaccine is intended.
- Phase 3 – Vaccine is given to several thousand people and tested for efficacy and safety.
How do we know whether the vaccine succeeded?
- During the third phase, participants either receive the vaccine or a placebo.
- The efficacy of the vaccine is determined by comparing the prevalence of infection in the group that was administered the vaccine with the one which received a placebo.
- The hypothesis that those in the vaccine group will be infected significantly less is thus tested.
What are the problems with Clinical Trials ?
- Clinical trials almost never succeed – Less than 10 percent of drugs that enter clinical trials are ever approved by the Food and Drug Administration.
- We have never released a coronavirus vaccine for humans before.
- Our record for developing an entirely new vaccine is at least four years.
- What are human challenge trials?
- Under human challenge trials, participants of both the vaccine group and placebo group upon consent are deliberately exposed to the infection
- Thus, they are “challenged” by the disease organism.
- Benefits – Saving of valuable time
- No wait for participants to contract the infection in real-world conditions.

- Controlled human challenge trials of SARS-CoV-2 vaccine candidates could accelerate the testing and potential rollout of efficacious vaccines.
- By replacing conventional Phase 3 testing of vaccine candidates, such trials may subtract many monthsfrom the process.
- Such testing would also require significantly less number of people than regular Phase 3 trials.
- First, do no harm
- is a fundamental principle of medicine throughout the world
The Ethical issues
- Human challenge studies have been conducted over hundreds of years and have contributed vital scientific knowledge that has led to advances in the development of drugs and vaccines.
- While human challenge trials are not new, they are usually carried out in developing medications for diseases which are considered less lethal, for which ‘therapeutic treatment’ is available and have been better understood by scientists over the years, such as malaria.
‘Volunteer’


- When conducted, human challenge studies should be undertaken with abundant forethought, caution, and oversight.
- The value of the information to be gained should clearly justify the risks to human subjects.

Scientific concerns
- Young people , too, are dying from COVID 19
- One concern is that given the volunteers are young and healthy, any trial results won’t be applicable across the population.
- After the challenge trial, we still wouldn’t necessarily know if older people respond in the same way as younger people to a vaccine.
Latest Burning Issues | Free PDF




















 WhatsApp
WhatsApp