Table of Contents


Name of the Vaccine
- The vaccine, ChAdOx1 nCoV-19 (also called AZD1222),
- Designed by Oxford and developed by AstraZeneca, the Anglo-Swedish pharma major.
- How does the Oxford-AstroZeneca vaccine work?
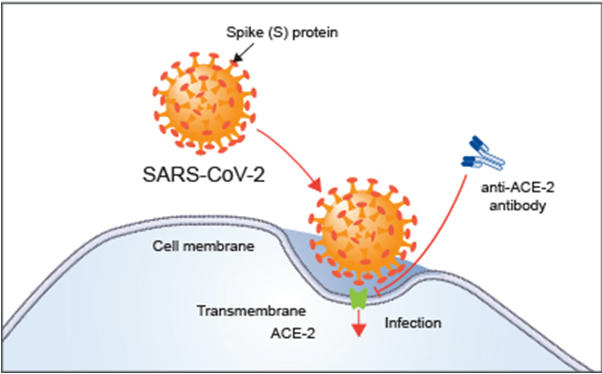
- ‘Spike protein’, allow the virus to penetrate cells & thereafter, multiply.
- The vaccine developed by Oxford and AstraZeneca, tries to build the body’s immunity against this spike protein.
- The idea is to create antibodies to fight this spiked surface so that the virus does not even have the chance to penetrate the cells.
The AstraZeneca uses different virus
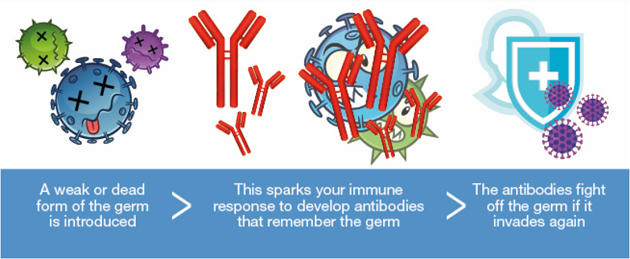
- The vaccine uses a different virus — in this case, a weakened version of a common cold virus (adenovirus) that infects chimpanzees — to carry just the code to make the spike protein, like a Trojan horse.
- The adenovirus, genetically modified so that it cannot replicate in humans, will enter the cell and release the code to make only the spike protein.
What the latest results show?

- The preliminary results from phase I/II trials of the vaccine, offered some promise by showing the vaccine was not only safe,
- But also seemed to build an immune response in the participants.
Dual immune response
- For one, it was found that being injected with the vaccine led to participants building neutralising antibodies.
- Another potential positive was that it increased the number of T cells — a type of white blood cell that protects the body from pathogens works to actively destroy infected cells.
Side effects
- The vaccine showed mild to moderate adverse reactions, including pain, feverish feelings, chills, muscle aches, headaches and malaise.
- The effects were reduced using prophylactic paracetamol, according to the study.
- Does it mean, vaccine will be ready in few months?
- While the results seem promising, it is important to remember this data is from early-stage clinical trials.
- This data cannot give clarity on questions like how long the antibodies will last in the body, an important factor in determining how effective the vaccine will be.
- This will require data from larger, phase III trials.

What happens next?
- Oxford and AstraZeneca have already begun phase III trials in Brazil, targeting 5,000 volunteers.
- A similar trial in South Africa is also expected to be underway.
Good new for India?
- Serum Institute of India, which has tied up with Oxford and AstraZeneca, plans to make “millions of doses” of the vaccine over the next three months.
- However, Serum will have to conduct phase III trials in India before the vaccine can be launched.
Latest Burning Issues | Free PDF





















 WhatsApp
WhatsApp